Our products

Customised user interfaces
Customised solutions for your medical device.
Best solution in every segment.
Our applications

Research cooperations
Ergonomic comfort, usability and wireless technology are "classic" research fields for steute Meditec. In addition, we are key players in the drive to implement an innovative SDC (Service-oriented Device Connectivity) interface for the interoperable OR of the future.
News & press releases
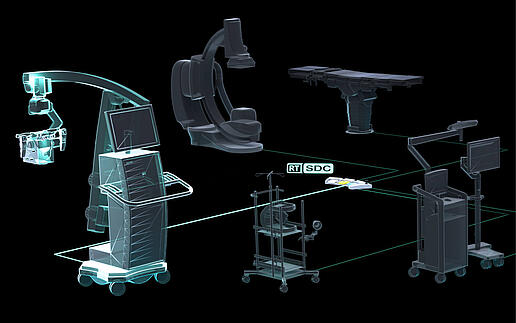
DMEA 2024 – steute Meditec and SurgiTAIX agree cooperation: joint development of Real-Time SDC for medical devices
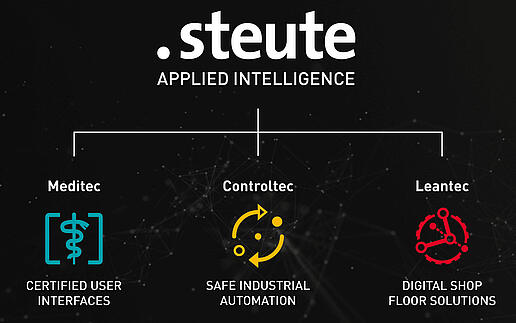
steute Technologies restructures its business fields

steute Technologies expands its Management
We look forward to meeting you!
-
-
-

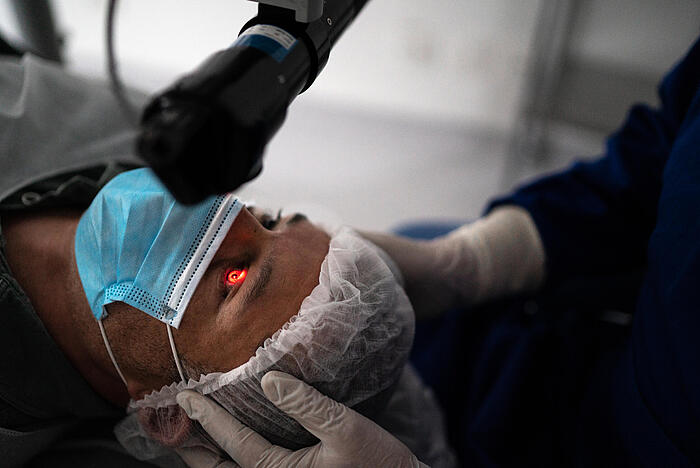
steute Meditec: the specialist for user interfaces in the OR
Many leading manufacturers of medical devices worldwide trust steute Meditec to develop and produce user interfaces for their devices expertly and competently. As a result they are safe in the knowledge that they will be receiving state-of-the-art technology, guaranteeing safe and intuitive operation of their top-level devices and, not least, profiting from the support which steute Meditec can offer them with approvals, certification and documentation.
- Wide range of standard controls
- Joint development of customised user interfaces
- Comprehensive portfolio of services, including testing, documentation and certification
- High level of research and development expertise