PocSpec
Modular Specialisations for Point-of-Care Medical DevicesThe "Modular Specialisations for Point-of-Care Medical Devices" (PoCSpec) project commenced in January 2019 and aims, on the basis of the ISO/ -IEEE 11073 directives, to develop standards which facilitate the integration of medical devices from specific disciplines, including especially complex sub-categories such as endoscopy or high-frequency surgery.
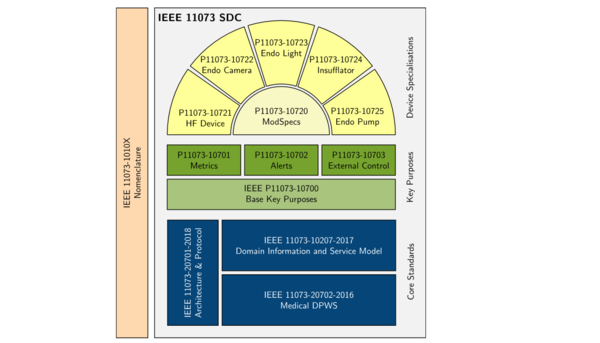
The PoCSpec partners are working on standards for the scope, structure and interpretation of data and services provided within the network, as well as for device behaviour. A key focus of the architecture currently under development is so-called ModSpecs: reusable modules for frequently occurring components in different devices, to be used for the simplification and standardisation of technical manuals. This will enable devices from different manufacturers to be monitored and controlled via the network.
The PoCSpec partners come from research backgrounds, but also from industry – especially from the fields of endoscopy and high-frequency surgery (including peripheral devices, e.g. for anaesthesis and insufflation). steute Meditec is involved in this project as an associated partner.
In brief
- Project start: 1st January 2019
- Project end: 30th June 2021
- Associated partner

Project partners
- Aesculap AG
- BOWA electronic GmbH & Co. KG
- Drägerwerk AG & Co. KGaA
- embeX GmbH
- Erbe Elektromedizin GmbH
- Institute for Applied Microelectronics and Data Engineering (IMD), University of Rostock
- Institute of Medical Informatics (IMI), University of Lübeck
- KARL STORZ SE & Co. KG
- OFFIS-Institut für Informatik
- Olympus Winter & Ibe GmbH
- Open Connections GmbH
- Richard Wolf GmbH
- Schölly Fiberoptic GmbH
- Söring GmbH
- steute Technologies GmbH & CO. KG
- SurgiTAIX AG
- Trusted Solutions Foundry, Inc.